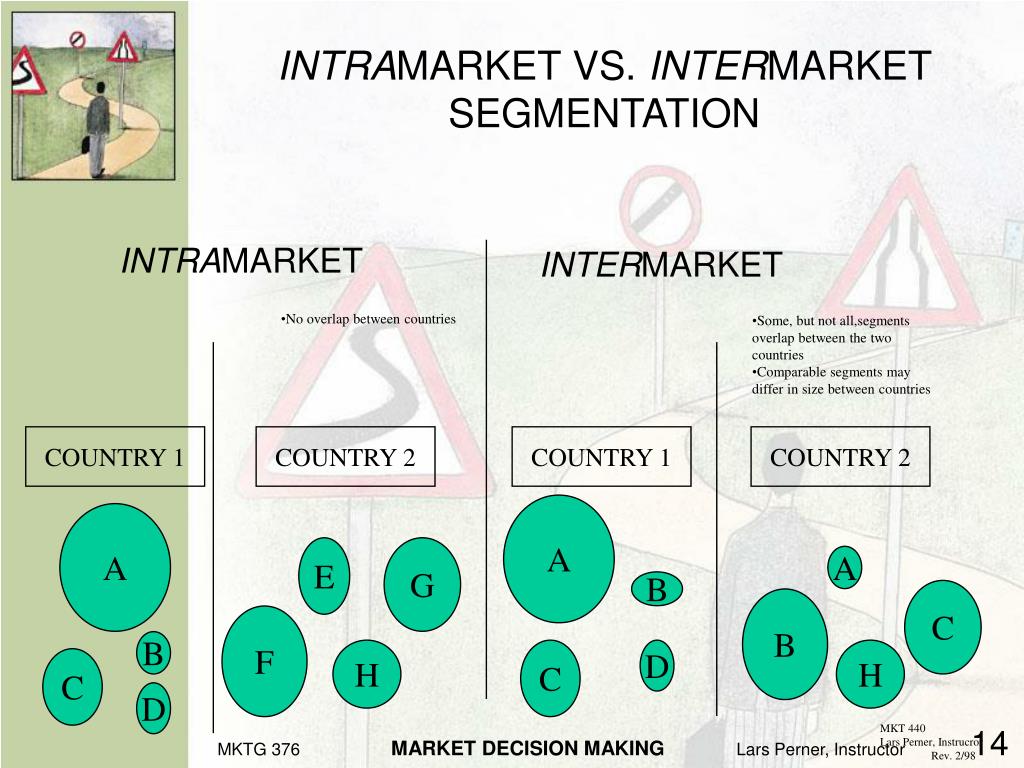
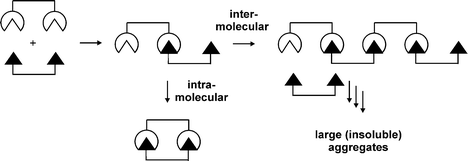
Inter Vs Intra Chemistry. Intra means within, this is one dipole Intermolecular is between two dipoles that make a bond and can be easily broken. Dipole-dipole, dipole-induced dipole, dispersion forces, hydrogen bonding are some of the examples for intermolecular forces. "How do I remember the difference between intra- and inter-molecular bonds?" It's a common question, and today, our Chemistry tutor solves it.
Types of Variations ● Parameters of transistors vary from: ○ Lot to lot (Inter process variation*) ○ Wafer to wafer (Inter process variation) ○ Die to die (Intra process variation) ○ Transistor to transistor (On Chip Variation) ●.
Chemistry Honors Podcast about Inter vs.
Inter- vs Intra-" "Inter-" and "intra-" are two prefixes which are commonly used in the English language. Hydrogen bonding is very important in the proper folding of a protein and they helps stabilize secondary protein structure. Intra Molecular Forces, made by Amy S., Kersee K., and Andrea C.